Pacing Non-Capture in a Child with Compound Heterozygous SCN5A Mutations
Apinya Bharmanee, James M Galas, Richard A Humes and Harinder R Singh
1Division of Pediatric Cardiology, Simitivej Hospital, Bangkok, Thailand
2Division of Pediatric Cardiology, The Carman and Ann Adams Department of Pediatrics, Children’s Hospital of Michigan, Detroit, MI, USA
3Department of Pediatrics, Department of Pediatrics, The Children’s Hospital of San Antonio, Baylor College of Medicine, San Antonio, TX, USA
- *Corresponding Author:
- Harinder R Singh
Department of Pediatrics, Department of Pediatrics
The Children’s Hospital of San Antonio, 315 N San Saba St., Suite 1135
San Antonio, TX 78207, USA
Tel: +12107044789
E-mail: Harinder.singh@christushealth.org
Received date: October 27, 2016; Accepted date: December 08, 2016; Published date: December 13, 2016
Citation: Bharmanee A, Galas JM, Humes RA, et al. Pacing Non-Capture in a Child with Compound Heterozygous SCN5A Mutations. Insights Pediatr Cardiol 2016, 1:1.
Copyright: © 2016 Bharmanee A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Mutations in the cardiac sodium channel gene SCN5A are linked to arrhythmias, cardiac conduction defects and cardiomyopathies. We report a 2 year old toddler with symptomatic sinus node dysfunction, negative family history, and increased left ventricular trabeculations. He underwent an unsuccessful attempt at transvenous pacemaker implantation, with no pacing capture at multiple ventricular and atrial sites. He required resuscitation for ventricular fibrillation, and was placed on extracorporeal membrane oxygenation (ECMO) support. Genetic analysis revealed compound heterozygous SCN5A loss-of-function and gain-offunction mutations, individually inherited from both his parents. In addition to the full spectrum of manifestations of SCN5A mutations, our patient also exhibited ventricular inexcitability.
Keywords
SCN5A mutation; Compound heterozygous; Pacing non-capture; Inexcitability
Case Report
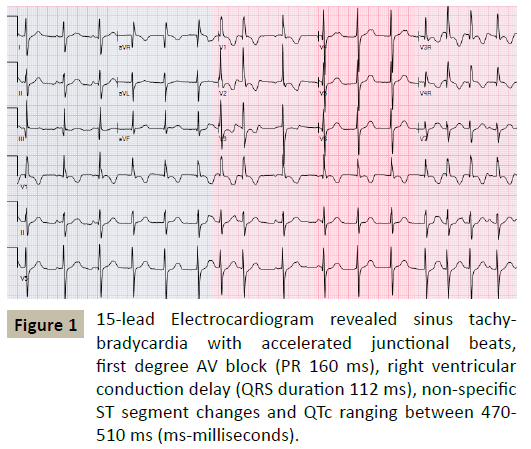
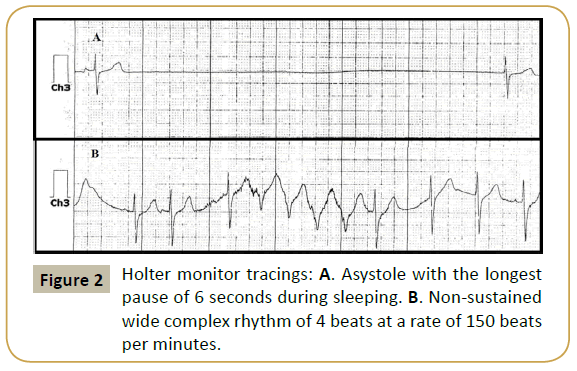
A 27-month old Caucasian boy with a previous history of syncope was referred to cardiology for evaluation of a heart murmur. Previously reported syncopal episodes had been ascribed to breath-holding spells. Family history was negative for sudden death, inherited arrhythmias or the need for pacemakers and defibrillators. His electrocardiograms revealed sinus tachy-bradycardia with accelerated junctional beats, first degree AV block, right ventricular conduction delay, nonspecific ST segment changes and QTc ranging between 470 - 510 milliseconds (Figure 1). Echocardiography demonstrated increased left ventricular trabeculations with non-compacted to compacted ratio of >2:1 with low normal systolic function. A 24 hour Holter monitor showed heart rates ranging 10-110 beats per minute with an average heart rate of 72 bpm, multiple pauses with the longest pause of 6 seconds and a run of nonsustained wide complex rhythm at a rate of 150 bpm (Figure 2). Pacemaker implantation was recommended and the family chose transvenous route of implantation. Cardiac MRI was performed as pacemaker implantation would preclude a study later. The patient underwent general anesthesia by a cardiac anesthetist utilizing sevofluorane, fentanyl and vecuronium during induction. The MRI reported prominent trabeculations in left ventricle however the patient did not meet the MRI criteria for LV noncompaction and no evidence of late gadolinium enhancement.
Sinus node function was studied before pacemaker implantation. Baseline electrocardiograms showed sinus bradycardia with intermittent junctional escape rhythm, sinus pauses and intermittent ventricular ectopy. The amplitude of the atrial electrogram was 0.2-0.5 millivolts indicative of atrial quiescence. After multiple attempts to pace the atrium from different sites at maximum output, sinus node recovery time was measured with intermittent capture. Sinus node was significantly depressed with a corrected junctional recovery time of 4.1 seconds. Autonomic blockade revealed an abnormal intrinsic heart rate of 77 bpm (expected 117 beats per minute), suggesting intrinsic sinus node dysfunction. In view of the evidence of atrial standstill and inexcitability, it was decided to implant a single chamber pacing system with a ventricular lead. Initial attempts to place a ventricular pacing lead (Medtronic 3830-49 cm lead) failed to capture at maximum output from multiple septal pacing sites (x7). The patient developed episodes of bradycardia, asystole and ventricular fibrillation requiring defibrillation and cardiopulmonary resuscitation (CPR). It was then decided to attempt implantation of the pacing lead in the atrium. However, after attempts at multiple atrial sites (x4), no atrial capture was elicited at maximal output. Repeat attempts to pace using a different pacing lead (Medtronic 5076-49 cm) in the ventricle and the atrium were also unsuccessful. Blood gases and electrolytes were stable throughout the procedure. Episodes of asystole and ventricular fibrillation requiring CPR and intermittent defibrillation continued. ECMO support with lidocaine, isoproterenol and dobutamine infusions was initiated during CPR. Echocardiography revealed no pericardial effusion. Even though cardiac function and rhythm stabilized there was evidence of significant neurologic impairment. Following serial neurological evaluations, cardiorespiratory support was withdrawn. The family refused an autopsy, but consented to genetic testing.
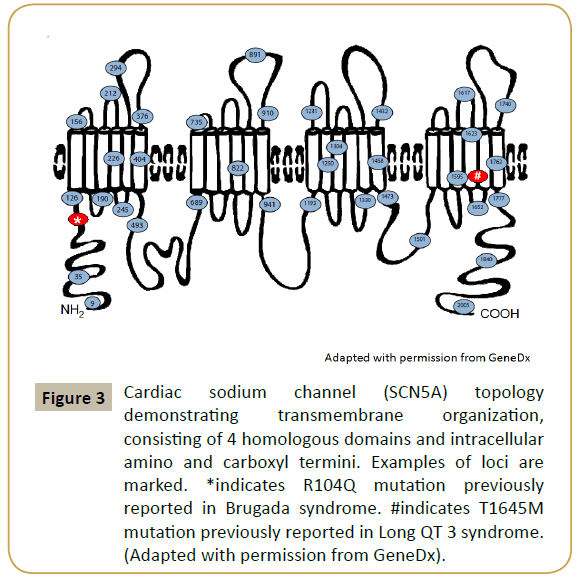
The patient was found to have two disease causing mutations on the SCN5A gene (R104Q and T1645M) as illustrated in Figure 3. Subsequently, the parents were tested for the identified mutations. The mother had the R104Q mutation in the SCN5A gene and the father was positive for the T1645M mutation in the SCN5A gene.
Figure 3: Cardiac sodium channel (SCN5A) topology demonstrating transmembrane organization, consisting of 4 homologous domains and intracellular amino and carboxyl termini. Examples of loci are marked. *indicates R104Q mutation previously reported in Brugada syndrome. #indicates T1645M mutation previously reported in Long QT 3 syndrome. (Adapted with permission from GeneDx).
Discussion
SCN5A gene, located on chromosome 3p21, encodes the alpha subunit of the voltage-gated cardiac sodium channel. It plays a major role in the initial depolarization phase, and determines the conduction velocity and excitability of cardiac myocytes [1,2]. SCN5A mutations have been associated with primary electrical and conduction defects such as Brugada syndrome, long QT3, atrial fibrillation, ventricular fibrillation, sudden infant death syndrome, atrial standstill, sick sinus syndrome and heart block [1,3-5] as well as dilated cardiomyopathy and left ventricular noncompaction [6,7]. Combination of mixed phenotypes can be seen even in the presence of a single SCN5A mutation [8,9].
SCN5A mutation expression is related to either loss-of-function or gain-of-function mutations. Both the form of mutations leads to alteration of electrical and mechanical properties of the cells. It is not well understood whether arrhythmias and structural defects are a direct effect of sodium current alterations, or merely secondary to long-standing cardiac abnormalities [9]. Presence of both loss and gain-of-function mutations in an individual may lead to varied phenotypic expressions which, so far, have not been defined.
SCN5A mutation is a prominent known cause for atrial inexcitability. Atrial inexcitability has been reported with compound heterozygous mutation of SCN5A cosegregated with Connexin 40 [10,11] and compound heterozygous loss-of-function mutations [12]. The preferential occurrence of atrial inexcitability in SCN5A mutation, rather than ventricle, may be related to the intrinsic differences between atrial and ventricular myocardium, such as liminal length for action potential propagation, sodium current densities and the speculation that the generation of ventricular action potentials in SCN5A mutation might depend upon sodium channel isoforms other than SCN5A expressed in ventricular tissue [13,14]. Alternatively, ventricle-specific metabolic or signal transduction events and alternative processing of SCN5A mRNA or accessory proteins other than β-1 and β-3 subunits may enable partial rescue of mutant SCN5A phenotypes in the ventricle but not the atrial myocardium [14].
Our patient had compound heterozygous mutations in the SCN5A gene. Individually, each mutation is a disease-causing mutation. Both are single base pair SCN5A mutations which lead to amino acid substitutions. The R104Q mutation is a loss-offunction mutation, previously reported in a patient with Brugada syndrome [15], whereas the T1645M mutation is a gain-offunction mutation, previously described in long QT3 syndrome patient [16]. The interaction between two pathogenic mutations in a single patient has not been previously reported. Our patient had symptomatic sinus node dysfunction, first degree AV block, right ventricular conduction delay, QT prolongation, atrial quiescence, both atrial and ventricular inexcitability, ventricular arrhythmia and suspicion for LV non compaction, which may also be regarded as an overlap syndrome. Intermittently elevated ventricular pacing thresholds has been reported in 3/11 patients in a case series with SCN5A loss-of-function mutations associated with poor pacemaker capture [17]. We postulate that the lossof- function and gain-of-function mutations together may have led to impairment in the action potential generation and/ or propagation in the ventricle as has been reported in the atrium, leading to pacing non-capture in the atrium and ventricle in our patient.
Our report suggests that genetic mutation(s) analysis may have a diagnostic benefit in the etiology of complex cardiac arrhythmias, particularly in the setting of known or suspected cardiomyopathy. Compound heterozygous SCN5A mutation(s) involving both lossof- function and gain-of-function mutation adds another facet to the spectrum of manifestations.
Acknowledgements
The authors wish to acknowledge and thank GeneDx for permission to use and adapt cardiac sodium channel SCN5A illustration.
References
- Bezzina CR, Rook MB, Wilde AA (2001) Cardiac sodium channel and inherited arrhythmia syndromes. Cardiovasc Res 49: 257-271.
- Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, et al. (2002) Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene SCN5A. Proc Natl Acad Sci USA 99: 6210-6215.
- Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, et al. (2000) Spectrum of mutations in long-QT syndrome genes: KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102: 1178-1185.
- Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, et al. (2003) Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest 112: 1019-1028.
- Remme CA, Bezzina CR (2010) Sodium channel (dys)function and cardiac arrhythmias. Cardiovasc Ther 28: 287-294.
- Hesse M, Kondo CS, Clark RB, Su L, Allen FL, et al. (2007) Dilated cardiomyopathy is associated with reduced expression of the cardiac sodium channel Scn5a. Cardiovasc Res 75: 498-509.
- Shan L, Makita N, Xing Y, Watanabe S, Futatani T, et al. (2008) SCN5A variants in Japanese patients with left ventricular noncompaction and arrhythmia. Mol GenetMetab 93: 468-474.
- Remme CA, Wilde AA, Bezzina CR (2008) Cardiac sodium channel overlap syndromes: different faces of SCN5A mutations. Trends Cardiovasc Med 18:78-87.
- Remme CA (2013) Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J Physiol 591: 4099-4116.
- Groenewegen WA, Firouzi M, Bezzina CR, Vliex S, van Langen IM, et al. (2003) A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res 92: 14-22.
- Makita N, Sasaki K, Groenewegen WA, Yokota T, Yokoshiki H, et al. (2005) Congenital atrial standstill associated with coinheritance of a novel SCN5A mutation and connexin 40 polymorphisms. Heart Rhythm 2: 1128-1134.
- Baskar S, Ackerman MJ, Clements D, Mayuga KA, Aziz PF (2014) Compound heterozygous mutations in the SCN5A-encoded Nav1.5 cardiac sodium channel resulting in atrial standstill and His-Purkinje system disease. J Pediatr 165: 1050-1052.
- Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, et al. (2002) An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci USA 99:4073-4078.
- Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, et al. (2003) An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci USA 100: 3507-3512.
- Levy-Nissenbaum E, Eldar M, Wang Q, Lahat H, Belhassen B, et al. (2001) Genetic analysis of Brugada syndrome in Israel: two novel mutations and possible genetic heterogeneity. Genet Test 5: 331-334.
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, et al. (1995) SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80: 805-811.
- Chiang DY, Kim JJ, Valdes SO, de la Uz C, Fan Y, et al. (2015) Loss-of-function SCN5A mutations associated with sinus node dysfunction, atrial arrhythmias and poor pacemaker capture. Circulation: Arrhythmia and Electrophysiology 8: 1105-1112.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences