Device Closure of Large Patent Ductus Arteriosus (PDA) in Young Infants: Analysis of Cases In a Bangladeshi Centre
Nurun Nahar Fatema*
SBP, Congenital and structural Interventionist, FCPS, FRCP (Edin), FACC, FSCAI, Combined Military hospital, Dhaka, Lab Aid Cardiac Hospital.
- *Corresponding Author:
- Nurun Nahar Fatema
SBP, Congenital and structural Interventionist, FCPS, FRCP (Edin), FACC, FSCAI, Combined Military hospital, Dhaka, Lab Aid Cardiac Hospital.
Email: colfatema@hotmail.com
Received date: August 01, 2022, Manuscript No. IPIPC-21-2631; Editor assigned date: August 03, 2022, PreQC No. IPIPC-21-2631 (PQ); Reviewed date: August 16, 2022, QC No. IPIPC-21-2631; Revised date: August 23, 2022, QI No. IPIPC-21-2631; Manuscript No. IPIPC-21-2631 (R); Published date: August 31, 2022, DOI: 10.36648/Insigh Pediatr Card.6.4.35
Citation: Fatema NN (2020) Device Closure of Large Patent Ductus Arteriosus (PDA) in Young Infants: Analysis of Cases In a Bangladeshi Centre Vol.S No.1: 1 DOI: 10.36648/IPIPC.6.4.35
Abstract
Aim: To assess the efficacy, outcome, challenges, and complications faced in device closure of infants upto one year of age.
Methods: All infants from December 2014 to December 2019 were included in the study. Out of 440 cases of percutaneous closure of patent ductusarteriosus (PDA) performed in the study period in all age groups, 170 cases were infants up to one year of age. PDA were closed with Duct occluderCeraTM,,Amplatzer duct occluder II (ADOII), Konar - Multifunctional Occluder (MFO), AmplatzerMuscular device and Cook detachable coils. Demographics included age, weight,gender and gestational age. Procedure and device data included narrowest size of PDA, association with PDA, haemodynamic significance, device type and size used and outcome. This is a retrospective review of the outcome and complications from records of catheterization laboratory and Echocardiography laboratory of a tertiary level cardiac hospital of Bangladesh.
Results: Median age was 7 months (10 daysto 12 months),median weight was 4.5 kg (1.8– 8.6 kg), 62.94% were female and 37.06% were male, 81.18% of infants were born after 37 weeks of gestation and 18.82 % were born before 37 weeks of gestation. Narrowest PDA diameter varied from 1.8 mm to 8.2 mm with median 06 mm. Pulmonary artery pressure varied from 40/20 mmHg to 105/70 mm Hg with median 70/40 mm Hg. Down syndrome was associated with 12.94% cases and congenital rubella syndrome in 8.82% cases. Most used device was 8X6 duct occluder. One hundred sixty-nine (99.41%) cases had complete closure and only one case (0.59%) required surgical closure due to embolization.
Conclusion: Transcatheter closure of PDA is a very safe, effective option of PDA closure in infants using various types of case appropriate devices in the market. In this series infants presented with heart failure, severe pulmonary hypertension, even with suprasystemicpulmonary pressure were cured completely immediately after closure.
Keywords
Patentductusarteriosus, LargetubularPDA,Infants, Outcome.
Abbreviations
ADOII-Amplatzer duct occlude II, MFO- Multifunctional Occluder. PDA- Patent ductusarteriosus, PAH- Pulmonary arterial hypertension, ASD – Atrial septal defect, MVP- Mitral valve prolapse, PM VSD- Perimembranous ventricular septal defect, PS- Pulmonary stenosis, CoA- Coarctation of aorta.
Introduction
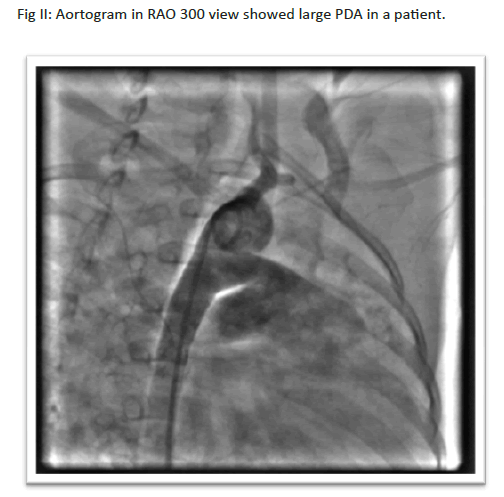
The incidence of isolated PDA has been estimated at 1:2000 to 1:5000 births which is about10 to 12 percent of all congenital heart disease1.Surgical closure of PDA was long been established but number reduced significantly as even neonatal large tubular PDAs are being closed by transcatheter technique safely and efficiently2 .Trans catheter closure of PDA using coil or device is an established method for older children and adults3. Since the first experience by Porstmann et al in 19674, technical improvement in device design and delivery system led to a revolutionary change in the field of intervention. Device closure in infants are challenging because most of them are symptomatic, failed to thrive (FTT) and body weight is less, need to close PDA for very large size which led tosevere pulmonary hypertension or heart failure. Sofavorable size device, hardware, avoidance of complications like mechanical obstruction to aorta or left pulmonary artery is a concern as these vessels are small size in comparison to the device size5,6. This study was conducted with an aim to see the outcome of transcatheter closure of PDA in infants upto one year of age with follow upto 4 months to 5 years.
Methods:
In this retrospective analysis, all infants upto one-year age were included from December 2014 to December 2019. Medical records from catheterization laboratory, Echo laboratory,postcatheterization ward and outpatient department were analyzed. Demographic information wascompiled along with echo and angiographic findings eg, haemodynamic data, shape and size of PDA, complications in cath lab, post cath area and follow up. The initial work up of the patient included physical examination to look for failure to thrive, association of genetic syndrome andsigns of heart failure. Echocardiography was performed to confirm diagnosis, measurement of size and shape of PDA , pulmonary artery pressure, direction of shunt, and association of other diseases like mitral valve prolapse (MVP),ventricular septal defect (VSD), atrial septal defect (ASD), coarctationof aorta (CoA), aortic stenosis (AS) etc. Echocardiographic assessment of shape and size of PDA was performed again one day prior to procedure and strategy was fixed for specific cases. Device size was determined for checking availability of device in catheterization laboratory (cath lab). All patients were deeply sedated with injection Ketamine, injection Midazolam, injection Phenobarbitone with minimum doses to avoid respiratory depression. During catheterization, pulmonary artery pressure, systemic to pulmonary artery flow ratio (QP:QS) were measured in cases where pulmonary pressure was more than two third systemic. PDA size at narrowest point and ampulla were measured and appropriate type of device and sizewere selected keeping in consideration the weight of the patient to match with delivery system. For tubular PDA, double disc devices were preferred. For very large tubular PDA, duct occluders were used by special technique. Aortic end of the duct was pulled inside PDA without opening completely inside aorta and a round onion like shape was given inside PDA shaft which retained it inside PDA by exerting pressure against PDA wall. Echo and fluoroscopy guide was taken during procedure. Patients were kept in observation for 24 hours after the procedure. Injection Ceftriaxone 50 mg/ kg intravenously (IV) was given during procedure and another dose was given after 24 hours.All patients were heparinized with 100 unit/kg body weight. No heparin was advised in post-cath observation wardunless there was pulse loss. Aspirin was not advised on discharge.Echocardiography was performed before discharge to checkforembolization, residual shunt etc. Follow up was given at 1, 3, 9,18 months and yearly thereafter for 3 years Devices used in this study were detachable coils (Cook Cardiology, USA), Heart &CeraLifetech duct occlude (Life tech Scientific, Schengen Co Ltd.), Konar-MFO-VSD occluder (Life tech Scientific, Schengen Co Ltd.), VSD muscular occluder (Life tech scientific), Cookin PDA occluder (Vascular innovation, Thailand) and ADO II (AGA Medical corporation, USA).
MS Excel was to do statistical analysis to find frequency andmedian. P value was determined if applicable.
Results
Table 1 showed demographic profile of the patient.Out of 170 cases, 48 (28.24%) were in 10 days to 06-month age group,122(71.76%) were in >06-month age group. Malewere 63(37.06%) and female were 107 (62.94%). Body weight of 22(12.94%) cases were between 1.8 - 03 kg. Seventy nine (46.47%) cases were between 3-5 kg and 69(40.59%) cases were more than 05 – 8.6 kg. The median weight was 4.5 kg. Gestational age at delivery was > 37 weeks in 138(81.18%) cases and <37 weeks in 32(18.82%) cases.
| S/No | Age | Number (%) | Median age |
|---|---|---|---|
| 01 | 10 days - 06 months | 48(28.24) | |
| >6 months – 01 year | 122(71.76) | 7 month | |
| 02 | Sex | ||
| Male | 63(37.06) | ||
| Female | 107(62.94) | ||
| 03 | Body Wt. | ||
| 1.8 - 03 kg | 22(12.94) | ||
| >03 – 05 kg | 79(46.47) | 4.5 kg | |
| > 05 kg-8.6 kg | 69(40.59) | ||
| 04 | Gestational age | ||
| Preterm (<37 weeks) | 32 (18.82) | 39 weeks. | |
| Term (>37 weeks) | 138 (81.18) |
Table 1: Demographic profile of patient
Table 2 showed association of PDA with other congenital heart diseases. Isolated PDA was seen in 129(75.88%) cases, association with ASD in 24 (14.12%) cases, coarctation of aorta in 3(1.76%) cases, PS in 12 (7.06%) cases, Pm VSD in 2 (1.18%) cases and MVP of AML in 28 (16.47%) cases.
| Disease | Number | Percentage |
|---|---|---|
| Isolated PDA | 129 | 75.88 |
| ASDII+ PDA | 24 | 14.12 |
| PDA+ COA | 3 | 1.76 |
| PDA+PS | 12 | 7.06 |
| PDA+ MVP AML | 28 | 16.47 |
| PDA + PM VSD | 2 | 1.18 |
Table 2: Association of other diseases with PDA.
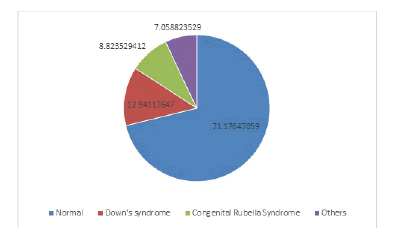
Fig I showed association with syndromes. Down syndrome was present t in 22 (12.94%) cases. Congenital Rubella Syndrome in 15 (8.82%) cases and uncommon syndrome in 12(7.06%) cases.
Table 3 showed angiographic measurement of narrowest part of PDA. PDA diameter was 1.8- 03mm in 42(24.70%) cases, >3-6mm in 54 (31.76%) cases, > 6 – 8.2 mm in 74 (43.53%) cases.
| Narrowest PDA diameter | No (%) | Median / Mode |
|---|---|---|
| 1.8- 3 mm | 42 (24.70) | 6 mm |
| > 3- 6mm | 54 (31.76) | |
| >6 mm- 8.2 mm | 74(43.53) | |
| Krichenko classification of shape | ||
| Type A | 42 (24.70) | Type C |
| Type B | 20 (11.76) | |
| Type C | 98 (57.65) | |
| Type D | 2 (1.18) | |
| Type E | 8 (4.71) | |
| Approach of closure | ||
| Arterial and venous | 146 (85.88) | Arterial and venous |
| Venous only | 14 (8.23) | |
| Arterial only | 10 (5.88) |
Table 3: Angiographic Data
According to Krichenkcoclassification, PDA type C was 98 (57.65%), type A was 42 (24.70%), type B was 20(11.76%), type D was 2(1.18) and type E was 8(4.71%) in number.
Arterial approach was used in 10 (5.88%) cases,venous approach in 14 cases (8.23%), arterial and venous approach in 146 (85.88%) cases.
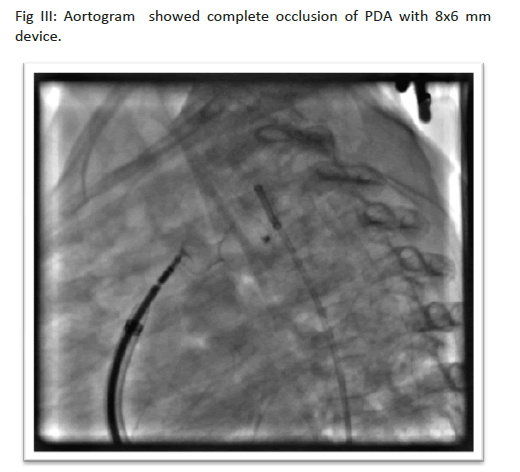
Table 4 showed device type and size as per distribution of diseases. Duct occluder were used in most of the cases, 8x6 mm Duct occluderdevice was the commonest(used in 31.76%cases). MFO in 1.17% cases, ADOII in 2.35% cases,VSD muscular device in 1.17% cases, PDA coil in 2.94% cases were used.
| Diseases | Type of devices used | Size | Number (%) | Total(%) |
|---|---|---|---|---|
| Isolated PDA | Duct occluder | 5x3 6x4 8x6 10x8 12x 10 14x12) |
7 (4.11) 22 (12.94) 52 (30.59) 36 (21.17) 26 (15.29) 3 (1.76) |
146(85.88%) |
| PDA Coil | 5x3 | 5 (2.94) | 5(2.94%) | |
| MFO | 6x4 | 2 (1.17) | 2(1.17%) | |
| ADOII | 6x4 | 4 (2.35) | 4(2.35%) | |
| VSD muscular device | 7mm, 8 mm | 2 (1.17) | 2(1.17%) | |
| PDA+ Pm VSD (VSD left open) |
Duct Occluder only | 10x8 12x10 |
1(0.58) 1 (0.58) |
2(1.16%) |
| PDA+ Coarctation of Aorta | Duct Occluder And Tyschak II Balloon |
10x8 8x6 |
2 (1.17) 1 (0.58) |
3(1.75) |
| PDA + PS | Duct Occluder And Tyschak II Balloon |
12x10 10x8 8x6 6x4 |
1 (0.58) 2 (1.17) 1 (0.58) 1 (0.58) |
5(2.94%) |
| Cook Coil and Tyshack II Balloon | 5x3 | 1 (0.58) | 1(0.58%) |
Table 4: Type of diseases & devices used.
Table 5 showed some angiographic variables. Median QP:QS ratio was 3:1, pulmonary pressure was 70/40/58 mmHg, fluoroscopy time was 12 min and median procedure time was 28 min
| Range | Median | |
|---|---|---|
| QP: QS | 1.5:1- 6.8:1 | 3:1 |
| Pulmonary Artery Pressure (mmHg) | 40/20/26- 150/70/96 (SBP/DBP/MBP) |
70/40/58 |
| Procedure time (min) | 20-65 | 28 |
| Fluoroscopy time (min) | 8-25 | 12 |
Table 5: Catheterization data
Table 6 showed complication and outcome up to one month follow up. Complete occlusion of PDA was noticed in 169(99.41%) cases. Tiny residual shunt was noticed in two cases(1.16%) after first 24 hours but no residual shunt was noticed in first follow up after one month. Transient arrhythmia were noticed in 3(1.76%) cases, pulmonary hypertensive crisis in 4 (2.35%) cases, temporary pulse loss in 5(2.94%) cases. One patient died immediately after dischargefrom hospital due to aspiration of milk. Right ventricular pressure remained significantly high in two (1.16%) cases in follow up, device was embolized in two cases (1.18%), one was retrieved, and coil occlusion performed in same setting. There was no intravascular hemolysis in any cases.
| Variables | Number (No) | Percent (%) |
|---|---|---|
| Cured completely | 169 | 98.82 |
| Pulmonary hypertensive crisis (transient) | 04 | 2.35 |
| Transient Arrhythmia | 03 | 1.76 |
| Temporary pulse loss | 05 | 2.94 |
| Embolization | 01 | 0.59 |
| Persistent raised right ventricular pressure | 01 | 0.59 |
| Referred to surgeon | 01 | 0.59 |
| Mortalityin cath lab or post cath ward | 0 | 0 |
| Death due to milk aspirationwithin 72 hours. | 01 | 0.59 |
| Residual shunt after one month follow up | 0 | 0 |
| Mechanical obstruction to aorta | 1 | 0.59 |
| Mild LPA stenosis | 2 | 1.18 |
Table 6: Complications and outcome up to first follow up.
Discussion: By now transcatheter closure for PDA has been established as first choice therapy in many centers.6 Despite the advancement of the procedure,there are limitations in closure of small children. The procedure was initially recommended only for children weighing over seven to eightkilograms, because size of the femoral vessels was relatively smaller than delivery system.7,8 In this study we had seen efficacy and outcome in infants up to one year of age. Few studies investigated transcatheter closure in preterm, neonates and in children less than 10 kg.9 Transcatheter closure of PDA is a minimally invasive therapy associated with low rate of adverse effect in children> 5 kg.10 In premature infant it was never tried earlier for fragility and concern regarding arterial / venous access. Suitable devices appropriate for neonatal PDA anatomy was also not available.11 But recently it has been established that it may be performed in newborn also.12 In our series (Table 1) lowest age was 10 days and highest was 12 months. Median age was 07 months. There were more female (62.94%)than male which is consistent with other studies2,3. Babies born preterm were 18.82% of the cohort which was a factor for significant number of patient in< 6 month age group because incidence of PDA is more in preterm babies13. A study showed incidence of PDA in preterm is 16 times more thanfull-term infants.2 Delayed closure of hemodynamically significant PDA in neonates lead to many complications14. Transcatheter closure is a better option for such cases and surgical risk can be avoided.
Association of PDA with other congenital anomalies correlates with other studies2. In one study association of MVP was maximum specially with cases where LV is moderately dilated or more2(Table 2).
Many of our cases were associated with Congenital Rubella syndrome(CRS) and Down syndrome (Figure 1). Incidence ofcongenital rubella syndrome is still high in Bangladesh because immunization against rubella only started couple of years ago and still most of the young girls and women of reproductive age are not immunized. Large tubular PDA and pulmonary artery stenosis are common association in this group of patient9.
The type ofdevices used in our series depended on the weight ofthe patient and shape and size ofPDA (Table 3). In fourteen cases, devices were deployed through venous approach. In four cases arteries were not accessible and in ten cases arteries were not cannulated intentionally to avoid complications of pulse loss in small infant. Only arterial approach was used when coil, MFO-Konar (Lifetech Scientific) and ADO II type devices were used. ADOII or MFO were used in neonates or very young infants through small delivery catheter like 5 French JR guide catheter for PTCA(Type C PDA, Krichenco classification)10, upto 12x10 size duct occluders were delivered through6 Frenchcookon delivery which is unique to allow large size devices,11. Wepreferred duct occluder(CeraTM) for young infants for its soft texture . For children more than 5 kg we used VSD muscular devices (B,C type of PDA) also along with duct occluder. In some cases, we pulled aortic endofCeraTM device inside PDA and formed a round onion shape which itself pressed against PDA wall and remained stable. The most used device size was 8x6 mm(31.76%) Cera TM of Lifetech Scientific(Table 4).
Challenges faced in early part of 2007, when this team started closing tubular PDA in young infant was size of the delivery system. Delivery system of 6French size from CookonVacular Innovation, Thailand was found suitable for its wide lumenand thin wall which allowed upto 12x10 size PDA devices to cross through it. A 2.8 kg infant of 6 months had large tubularPDA with heart failure and was closed with 12x10 duct occluder at that time .Successful closure of that young infant through small delivery system encouraged the team to close PDA in young infant routinely thereafter. Fluoroscopy and procedure time were 8 to 25 minutes and 20 to 65 minutes respectively with median of 12 and 28 minutes (Table 5).
In this cohort, our median QP:QS, pulmonary artery pressure, fluoroscopy time and procedure time corelate with other studies11,12. As the indication of closure was large and tubular PDA in young infant in this study group, all of the cases had pulmonary hypertension of various grade (Table 5).The majority had (74.12%) severe pulmonary hypertension1 which led to an early closure of the shunt in young infant. Fluoroscopy and procedure time of the study reduced significantly with learning curve of the team.
Our time was much less than a study conducted in Sohag University Hospital3. The reason for this was members of intervention team had long experience on the basis of which decisions were taken quickly , any types of hemodynamic instabilities were sorted out at the earliest chance and strategy of intervention was planned on day before procedure and trollies were prepared accordingly byscrub nurse with all necessary disposables.
In this study out of 170 cases 169 (99.09%) caseswere cured completely (Table 6) which is similar toanother study,3. Major complications were embolization of device in two cases, one retrieved and closed with coil, another sent to operation theater for surgical closure. Our study correlates with other studies14-17. Small residual shuntswere seen in two casesduring discharge but disappeared at one month follow up. RV pressure came down to normal in all cases except one. One patient expired from aspiration of milk on the day of discharge. In this study, no problem was encountered with delivery system, we had multiple option of delivery system of MFO, ADOII, 6 FrenchCookon PDA delivery system to cover upto 12x10 size devices in young infants. Possibility of mechanical obstruction to aorta and left pulmonary artery were excluded by measuring withdrawal pressure gradient before release of deviceand by echo CW Doppler. We have not seen any difference in outcome with different devices but availability of double disc devices like MFO with various sizes enabled us to include more newborn and young infants in later part of the study period. Even a new device just arrived in the market named Amplatzer Piccolo Device (Abbott Structural Heart, Plymouth, MN, USA) for newborn more than 700 gram, but this device is not available in our region yet. Theoutcome of this study in term of success and complications is similar to other studies18-20.
Conclusion: Device closure of PDA in neonates and young infant has become very safe with innovation of newer generation devices. It has becomethe first choice of therapy for PDA closure in many centers like us. In last 15years we have referred only 5 cases to cardiac surgeons. So this study has shown excellent outcome of young infant for percutaneous closure of PDA with very minimum complications.
References
- N NFatema . Device Closure of a Large Patent DuctusArteriosus with Severe Pulmonary Hypertension Using Amplatzer Device: A Case Report. ASEAN Heart Journal 2006;14(1):12-15.
- Safaa Ali, Amel El Sisi. Transcatheter closure of patent ductusarteriosus in children weighing 10 kg or less: Initial experience at Sohag University Hospital. J Saudi Heart Assoc. 2016 Apr; 28(2): 95–100.
- Ullah M, Sultan M, Akhtar K, Sadiq N, Akbar H. Percutaneous transcatheter PDA device closure in infancy. J Coll Physicians Surg Pak 2014; 24(8): 581-5
- Porstmann W, Wierny L, Warnke H. Closure of persistent ductusarteriosus without thoracotomy. Ger Med Mon1967;12:259–261.
- Al- Ata J, Arif AM, Hussain A, Kouatli A A, Jalal MO. The Efficacy and safety of Amplatzer duct occluder in young children and infants. Cardiol Young 2005; 15:279-285.
- Ewert P. Challenges encountered during closure of patent ductusarteriosus. PediatrCardiol 2005;26:224–229.
- Butera G, De Rosa G, Chessa M, Piazza L, Delogu A, Frigiola A. Transcatheter closure of persistent ductusarteriosus with the Amplatzer duct occluder in very young symptomatic children. Heart 2004;90:1467–1470.
- NNF Begum, AU Ahmed. Acute Intra Vascular Haemolysis after Coil Occlusion of Patent DuctusArteriosus by Detachable Coil - A Case Report.BCPS Journal 2003;21:151-153.
- N NFatema. Device Closure of a Large Patent DuctusArteriosus with SeverePulmonary Hypertension: A Case Report.Bangladesh heart J 2005;20(2):73-76.
- 10.KrichenkoA,BensonLN,Burrows P, Moes CA, McLaughlin P, Freedom RM.Angiographic classification of the isolated, persistently patent ductusarteriosus and implications for percutaneous catheter occlusion.Am J Cardiol. 1989;63(12):877-880.doi:10.1016/0002-9149(89)90064-7.
- Kumar SM1, Subramanian V, Bijulal S, Krishnamoorthy KM, Sivasankaran S, TharakanJA.Percutaneous closure of a moderate to large tubular or elongated patent ductusarteriosus in children younger than 3 years: is the ADO II appropriate PediatrCardiol 2013;34(7):1661-7.
- Park Y.A, Kim N.K, Park S.J, Yun B.S, Choi J.Y, Sul J.H. Clinical outcome of transcatheter closure of patent ductusarteriosus in small children weighing 10 kg or less. Korean j Pediatr 2010; 53:1012-1017.
- Jang G.Y, Son C.S, Lee J.W, Lee J.Y, Kim S.J. Complications after transcatheter closure of patent ductusarteriosus. J Korean Med Sci. 2007;22:484–490.
- Pruetz JD, Carroll C, Trento LU. Outcome of critical congenital heart disease requiring emergent neonatal cardiac intervention.PrenatDiagn. 2014;34: 1127-1132.
- Masura J, Tittel P, Gavora P, Podnar T. Long-term outcome of transcatheter patent ductusarteriosus closure using Amplatzer duct occluders.Am Heart J. 2006;151:755.e7–755.e10.
- Pass R.H, Hijazi Z, Hsu D.T, Lewis V, Hellenbrand W.E. Multicenter USA Amplatzerpatentductusarteriosus occlusion device trial: Initial one-year results.J Am CollCardiol. 2004;44:513–519.
- Behjati-Ardakani M, Behjati-Ardakani M.A, Hosseini S.H, Noori N. Long-term results of transcatheterclosure of patent ductusarteriosus in infants using Amplatzer duct occluder. Iran J Pediatr. 2013;23:411–416.
- Bergersen L, Marshall A, Gauvreau K, Beekman R, Hirsch R, Foerster S. Adverse event rates in congenital cardiac catheterization—a multi-center experience. Catheter CardiovascInterv. 2010;75:389–400.
- N NFatema. Device closure of patent ductusarteriosus in complicated patient.JAFMC 2011; 7(1):43-45.
- Kim Y, Choi J.Y, Lee J.K, Sul J.H, Lee S.K, Park Y.H.Mid-term result of the transcatheter occlusion of patent ductusarteriosus with Duct-Occlud device and procedure-related problems. Korean J Pediatr. 2004;47:36–43.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences